Decoy-resistant IL-18: a new paradigm in cytokine therapy
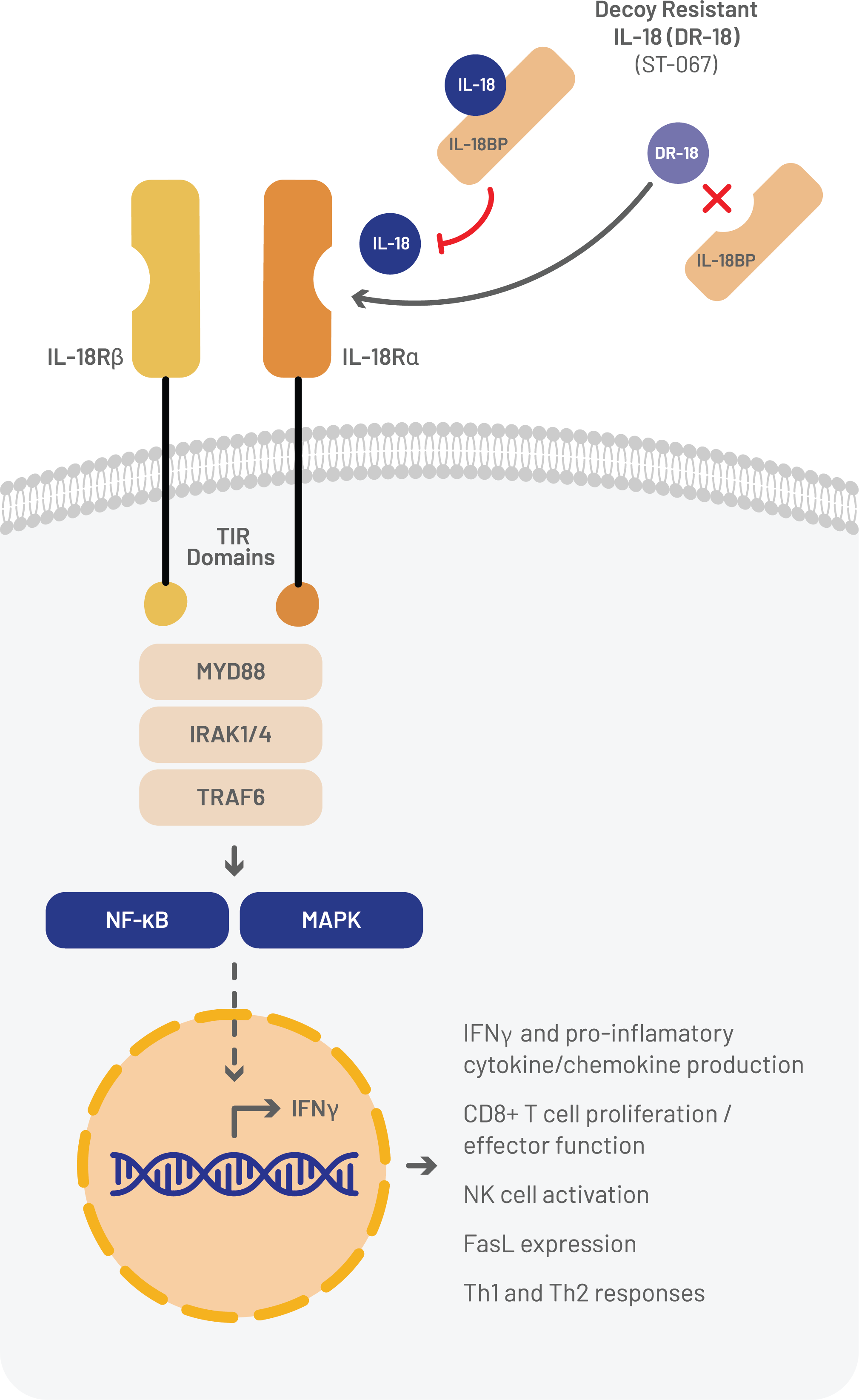
Interleukin-18 (IL-18) is an inflammatory cytokine with powerful immunostimulatory activities on key anti-tumor immune cells: tumor infiltrating lymphocytes (TILs) and natural killer (NK) cells. IL-18’s ability to activate both the innate and adaptive branches of the immune system indicates that it could exert a broad spectrum of activity against both immunogenic, or “hot” tumors, as well as “cold” tumors that are resistant to immunotherapy. However, previous clinical experience with recombinant IL-18 therapy revealed a surprising paradox: despite its well-established immunostimulatory activities, IL-18 therapy was ineffective, even in cancer types that have historically responded to immunotherapeutic agents.
Research led by Simcha’s founder Dr. Aaron Ring revealed the basis for natural IL-18’s weak anti-cancer activity. Tumors produce high levels of an IL-18 jamming signal, a “decoy receptor” called IL-18BP. To overcome this problem, Simcha has engineered the first “decoy-resistant” IL-18 variant, ST-067, that is completely impervious to IL-18BP and can maintain strong immune stimulation in the tumor microenvironment. In preclinical tumor studies, decoy-resistant IL-18 demonstrated robust anti-tumor activity by itself and in combination with immune checkpoint inhibitors such as anti-PD-1.
ST-067 is now under evaluation in a Phase 1a/2 clinical trial in patients with diverse solid tumors who have progressed on existing immunotherapeutic agents, including one cohort combining ST-067 with pembrolizumab (Keytruda®). We are also collaborating with researchers at the Fred Hutchinson Cancer Research Canter and separately at the University of Washington to study ST-067 in hematological cancers.
Publications
Partnering
We are seeking collaborations with industry and academia to fully explore the clinical utility of IL-18 in oncology and other indications. We welcome opportunities to share ideas.
Please reach out to partnering@simchatherapeutics.com to engage.
Our Partners
| Program | Preclinical | Phase 1 |
|---|---|---|
| ST-067 | Monotherapy Solid Tumors | ||
| ST-067 | Pembrolizumab Solid Tumors | ||
| ST-067 | Teclistamab Multiple Myeloma | ||
| ST-067 | Monotherapy Acute Myeloid Leukemia or Myelodysplastic Syndrome | ||
| DR-18 Armored CAR T(s) Oncology | ||
| ST-067 | Combination Therapies Undisclosed | ||
| Cytokine Therapeutics Undisclosed |
| Program | Stage |
|---|---|
| ST-067 | Monotherapy | Phase 1 |
| ST-067 | Pembrolizumab | Phase 1 |
| ST-067 | Teclistamab | Phase 1 |
| ST-067 | Monotherapy | Phase 1 |
| DR-18 Armored CAR T(s) | Preclinical |
| ST-067 | Combination Therapies | Preclinical |
| Cytokine Therapeutics | Preclinical |



